Research Area F - Publications 2021
28-Jun-2021
Pharmaceuticals 2021, 14, 624, https://doi.org/10.3390/ph14070624
Pharmaceuticals, online article

Inhibition of protein–DNA interactions represents an attractive strategy to modulate essential cellular functions. We reported the synthesis of unique oligoamide-based foldamers that adopt single helical conformations and mimic the negatively charged phosphate moieties of B-DNA. These mimics alter the activity of DNA interacting enzymes used as targets for cancer ...
22-Jan-2021
Chem. Sci., 2021,12, 3743-3750, DOI: 10.1039/d0sc06060g
Chem. Sci.,, online article

The selective binding properties of a 13-mer oligoamide foldamer capsule composed of 4 different aromatic subunits are reported. The capsule was designed to recognize dicarboxylic acids through multiple-point interactions owing to a combination of protonation/deprotonation events, H-bonding, and geometrical constraints imparted by the rigidity of the foldamer ...
21-Jan-2021
Angew. Chem. Int. Ed. 2021, 60, 8380 –8384, https://doi.org/10.1002/anie.202100349
Angew. Chem. Int. Ed., online article
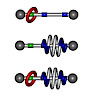
The design and synthesis of a novel rotaxane/foldaxane hybrid architecture is reported. The winding of an aromatic oligoamide helix host around a dumbbell‐shaped thread‐like guest, or axle, already surrounded by a macrocycle was evidenced by NMR spectroscopy and X‐ray crystallography. The process proved to depend on the position of the macrocycle along the axle ...
06-Nov-2020
Angew. Chem. Int. Ed. 2021, 60, 2574 –2577, doi.org/10.1002/anie.202014670
Angew. Chem. Int. Ed., online article
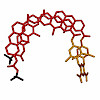
The orchestration of ever larger conformational changes is made possible by the development of increasingly complex foldamers. Aromatic sheets, a rare motif in synthetic foldamer structures, have been designed so as to form discrete stacks of intercalated aromatic strands through the self‐assembly of two identical subunits. Ion‐mobility ESI‐MS confirms the ...