Research Area B - Publications 2007
21-Dec-2007

The amoeba Dictyostelium discoideum possesses genes for 13 different kinesins. Here we characterize DdKif3, a member of the Kinesin-1 family. Kinesin-1 motors form homodimers that can move micrometer-long distances on microtubules using the energy derived from ATP hydrolysis. We expressed recombinant motors in Escherichia coli and tested them in different in ...
29-Nov-2007
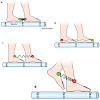
The motor protein kinesin ‘walks’ by alternately advancing its two motor structural domains. A cutting-edge, single-molecule fluorescencetechnique reveals further details of this stepping mechanism. Suppose that walking required energy input in the form of, say, one Gummi bear for every step. In what position would you pop the next Gummi bear into your mouth? ...
26-Oct-2007

In a recent issue of Molecular Cell, Dollins et al. (2007) present the crystal structure of Grp94, which highlights the similarity between Grp94 and Hsp90 and provides insight into the resting state of Grp94 and potentially other Hsp90 family members.
26-Oct-2007

In a recent issue of Molecular Cell, Dollins et al. (2007) present the crystal structure of Grp94, which highlights the similarity between Grp94 and Hsp90 and provides insight into the resting state of Grp94 and potentially other Hsp90 family members.
09-Oct-2007
JBC, 2007, published on 09.10.2007
www.jbc.org, online article

Grp94, the Hsp90 paralogue of the endoplasmic reticulum (ER), plays a crucial role in protein secretion. Like cytoplasmic Hsp90, Grp94 is regulated by nucleotide binding to its N-terminal domain. However, the question whether Grp94 hydrolyses ATP was controversial. This sets Grp94 apart from other members of the Hsp90 family where a slow but specific turnover of ...
01-Oct-2007
the FEBS Journal, 2007, 274, 5043–54 published on 01.10.2007
www.febsjournal.org

The import of proteins destined for the intermembrane space of chloroplasts has not been investigated in detail up to now. By investigating energy requirements and time courses, as well as performing competition experiments, we show that the two intermembrane space components Tic22 and MGD1 (E.C. 2.4.1.46) both engage the Toc machinery for crossing the outer ...