MacroH2A1.1 regulates mitochondrial respiration by limiting nuclear NAD+ consumption
09-Oct-2017
Nature Structural & Molecular Biology, volume 24, 902–910, doi:10.1038/nsmb.3481
Nature Structural & Molecular Biology, online article
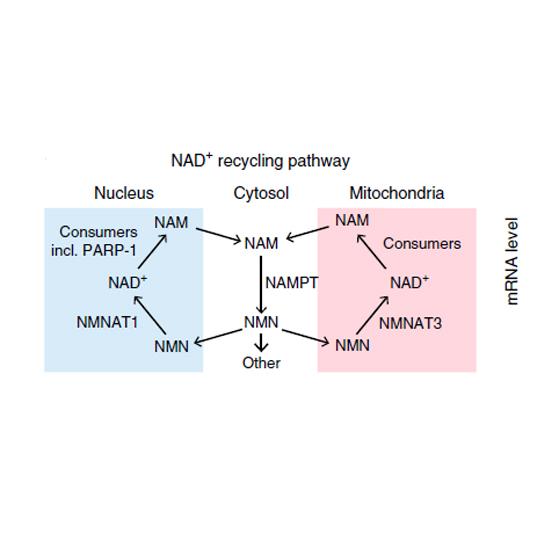
Histone variants are structural components of eukaryotic chromatin that can replace replication-coupled histones in the nucleosome. The histone variant macroH2A1.1 contains a macrodomain capable of binding NAD+-derived metabolites. Here we report that macroH2A1.1 is rapidly induced during myogenic differentiation through a switch in alternative splicing, and that myotubes that lack macroH2A1.1 have a defect in mitochondrial respiratory capacity. We found that the metabolite-binding macrodomain was essential for sustained optimal mitochondrial function but dispensable for gene regulation. Through direct binding, macroH2A1.1 inhibits basal poly-ADP ribose polymerase 1 (PARP-1) activity and thus reduces nuclear NAD+ consumption. The resultant accumulation of the NAD+ precursor NMN allows for maintenance of mitochondrial NAD+ pools that are critical for respiration. Our data indicate that macroH2A1.1-containing chromatin regulates mitochondrial respiration by limiting nuclear NAD+ consumption and establishing a buffer of NAD+ precursors in differentiated cells.