Suv4-20h Abrogation Enhances Telomere Elongation during Reprogramming and Confers a Higher Tumorigenic Potential to iPS Cells
12-Oct-2011
Plos, 2011, doi:10.1371/journal.pone.0025680, 6(10): e25680 published on 12.10.2011
Plos, online article
Plos, online article
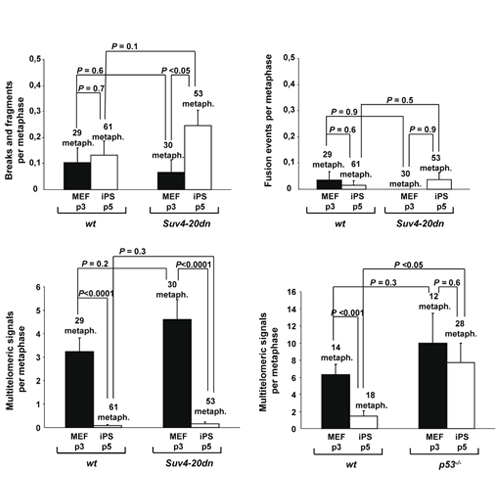
Reprogramming of adult differentiated cells to induced pluripotent stem cells (iPS) cells has been achieved by over-expression of specific transcription factors. Nuclear reprogramming induces a series of profound changes at the telomeres of the parental differentiated cells, including a telomerase-dependent telomere elongation and the remodeling of telomeric chromatin. In particular, iPS cells show a decreased density of H4K20me3 heterochromatic mark at telomeres compared to the parental cells. Suv4-20h1 and Suv4-20h2 histone methytransferases (HMTases) are responsible for the trimethylation of H4K20 at telomeres, as cells deficient for both HMTases show decreased levels of H4K20me3 at telomeric chromatin. Here, we set to address the role of the Suv4-20h enzymes in telomere reprogramming by generating bona-fide iPS cells from mouse embryonic fibroblasts (MEFs) double null for both HMTases (Suv4-20dn MEFs). We found that Suv4-20h deficiency enhances telomere elongation during reprogramming without altering their ability to protect the chromosome ends or the efficiency of reprogramming. Moreover, teratomas generated from Suv4-20dn iPS cells also have elongated telomeres and an increased growth rate when compared to wild-type controls. These results indicate that abrogation of Suv4-20h enzymes and loss of heterochromatic mark H4K20me3 at telomeric heterochromatin facilitates telomere reprogramming and provides an increased tumorigenic potential to the resulting iPS cells.