Research Area B - Publications 2009
22-Dec-2009
Trends in Biochemical Sciences, online article

B cells use unconventional strategies for the production of a seemingly unlimited number of antibodies from a very limited amount of DNA. These methods dramatically increase the likelihood of producing proteins that cannot fold or assemble appropriately. B cells are therefore particularly dependent on ‘quality control’ mechanisms to oversee antibody production. ...
10-Dec-2009
J. Biol. Chem., online article
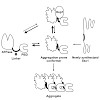
The co-chaperone Hep1 is required to prevent the aggregation of mitochondrial Hsp70 proteins. We have analyzed the interaction of Hep1 with mitochondrial Hsp70 (Ssc1) and the determinants in Ssc1 which make it prone to aggregation. The ATPase and the peptide binding domain (PBD) of Hsp70 proteins are connected by a linker segment that mediates interdomain ...
01-Dec-2009
Infection and Immunity, online article

Preexisting antivector immunity can severely compromise the ability of Salmonella enterica serovar Typhimurium live vaccines to induce protective CD8 T-cell frequencies after type III secretion system-mediated heterologous protein translocation in orally immunized mice. To circumvent this problem, we injected CpG DNA admixed to the immunodominant p60217-225 ...
01-Nov-2009
Mol. Plant, 2009, 2(6), 1410-1424, doi:10.1093/mp/ssp095 published on 01.11.2009
Molecular Plant, online article

All members of the YidC/Oxa1/Alb3 protein family are evolutionarily conserved and appear to function in membrane protein integration and protein complex stabilization. Here, we report on a second thylakoidal isoform of Alb3, named Alb4. Analysis of Arabidopsis knockout mutant lines shows that Alb4 is required in assembly and/or stability of the CF1CF0–ATP ...
12-Oct-2009
Fungal Genetics and Biology, online article

GDP-mannose:inositol-phosphorylceramide (MIPC)-derived glycosphingolipids are important pathogen-associated molecular patterns (PAMP) of Candida albicans and according to recently published data also of Aspergillus fumigatus. MIPC transferases are essential for the synthesis of MIPC, but have so far been studied only in Saccharomyces cerevisiae and C. albicans. ...
09-Oct-2009
J. Biol. Chem., online article

Hsp90 is an ATP-dependent molecular chaperone which assists the maturation of a large set of target proteins. Members of the highly conserved Hsp90 family are found from bacteria to higher eukaryotes, with homologues in different organelles. The core architecture of Hsp90 is defined by the N-terminal ATP-binding domain, followed by the middle domain and the ...
09-Oct-2009
J. Mol. Biol., online article

Antibodies are modular proteins consisting of domains that exhibit a β-sandwich structure, the so-called immunoglobulin fold. Despite structural similarity, differences in folding and stability exist between different domains. In particular, the variable domain of the light chain VL is unusual as it is associated with misfolding diseases, including the pathologic ...
29-Sep-2009

Translocation of nuclear-encoded preproteins across the inner envelope of chloroplasts is catalyzed by the Tic translocon, consisting of Tic110, Tic40, Tic62, Tic55, Tic32, Tic20, and Tic22. Tic62 was proposed to act as a redox sensor of the complex because of its redox-dependent shuttling between envelope and stroma and its specific interaction with the ...
02-Sep-2009
Molecular Plant, online article

OBG-like GTPases, a subfamily of P-loop GTPases, have divers and important functions in bacteria, including initiation of sporulation, DNA replication, and protein translation. Homologs of the Bacillus subtilis spo0B GTP-binding protein (OBG) can be found in plants and algae but their specific function in these organisms has not yet been elucidated. Here, it is ...
01-Sep-2009

In plant cells calcium-dependent signaling pathways are involved in a large array of biological processes in response to hormones, biotic/abiotic stress signals and a variety of developmental cues. This is generally achieved through binding of calcium to diverse calcium-sensing proteins, which subsequently control downstream events by activating or inhibiting ...
26-Aug-2009

In eukaryotic cells, dozens to hundreds of different mRNAs are localized by specialized motor-dependent transport complexes. One of the best-studied examples for directional mRNA transport is the localization of ASH1 mRNA in Saccharomyces cerevisiae. For transport, ASH1 mRNA is bound by the unusual RNA-binding protein She2p. Although previous results indicated ...
21-Aug-2009
EMBO reports, online article

Heat shock protein 90 (Hsp90) is an abundant, dimeric ATP-dependent molecular chaperone, and ATPase activity is essential for its in vivo functions. S-nitrosylation of a residue located in the carboxy-terminal domain has been shown to affect Hsp90 activity in vivo. To understand how variation of a specific amino acid far away from the amino-terminal ATP-binding ...
19-Aug-2009
Biochim. Biophys. Acta, online article
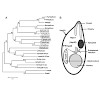
Small heat shock proteins (sHsps) are ubiquitous molecular chaperones which prevent the nonspecific aggregation of non-native proteins. Five potential sHsps exist in the parasite Toxoplasma gondii. They are located in different intracellular compartments including mitochondria and are differentially expressed during the parasite's life cycle. Here, we analyzed ...
19-Aug-2009
J. Mol. Biol., online article

Efficient formation of specific intermolecular interactions is essential for self-assembly of biological structures. The foldon domain is an evolutionarily optimized trimerization module required for assembly of the large, trimeric structural protein fibritin from phage T4. Monomers consisting of the 27 amino acids comprising a single foldon domain subunit ...
27-Jul-2009

alpha-Crystallins are molecular chaperones that protect vertebrate eye lens proteins from detrimental protein aggregation. alpha B-Crystallin, 1 of the 2 alpha-crystallin isoforms, is also associated with myopathies and neuropathological diseases. Despite the importance of alpha-crystallins in protein homeostasis, only little is known about their quaternary ...
07-Jul-2009
FEBS Lett., 2009, 583(13), 2237-2243, doi:10.1016/j.febslet.2009.05.053 published on 07.07.2009
FEBS Letters, online article

The mitochondrial dynamin-like GTPase Mgm1 exists as a long (l-Mgm1) and a short isoform (s-Mgm1). They both are essential for mitochondrial fusion. Here we show that the isoforms interact in a homotypic and heterotypic manner. Their submitochondrial distribution between inner boundary membrane and cristae was markedly different. Overexpression of l-Mgm1 exerts a ...
06-Jul-2009
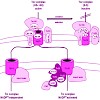
The import of nuclear-encoded preproteins is necessary to maintain chloroplast function. The recognition and transfer of most precursor proteins across the chloroplast envelopes are facilitated by two membrane-inserted protein complexes, the translocons of the chloroplast outer and inner envelope (Toc and Tic complexes, respectively). Several signals have been ...
01-Jul-2009

Members of the AAA+-ATPase superfamily (ATPases associated with various cellular activities) are found in all kingdoms of life and they are involved in very diverse cellular processes, including protein degradation, membrane fusion or cell division. The Arabidopsis genome encodes approximately 140 different proteins that are putative members of this superfamily, ...
30-Jun-2009

Chloroplast biogenesis in angiosperm plants requires the light-dependent transition from an etioplast stage. A key factor in this process is NADPH:protochlorophyllide oxidoreductase A (PORA), which catalyzes the light-dependent reduction of protochlorophyllide to chlorophyllide. In a recent study the chloroplast outer envelope channel OEP16 was described to be ...
15-Jun-2009
J. Cell Biol., 2009, 185(6), 1047-1063, doi:10.1083/jcb.200811099 published on 15.06.2009
J. Cell Biol., online article

Crista junctions (CJs) are important for mitochondrial organization and function, but the molecular basis of their formation and architecture is obscure. We have identified and characterized a mitochondrial membrane protein in yeast, Fcj1 (formation of CJ protein 1), which is specifically enriched in CJs. Cells lacking Fcj1 lack CJs, exhibit concentric stacks of ...
12-Jun-2009

A prerequisite for antibody secretion and function is their assembly into a defined quaternary structure, composed of two heavy and two light chains for IgG. Unassembled heavy chains are actively retained in the endoplasmic reticulum (ER). Here, we show that the CH1 domain of the heavy chain is intrinsically disordered invitro, which sets it apart from other ...
29-May-2009
http://www.lsm.bio.lmu.de/

In order to promote future scientists the best way we can, CIPSM decided to form an own Graduate School. The Graduate School Life Science Munich (LSM) of the Ludwig-Maximilians-University Munich (LMU) offers an international doctoral program in life sciences covering areas of anthropology, biochemistry and cell biology, ecology, evolution, genetics, microbiology, ...
01-Apr-2009
Biological Chemistry, online article

Molecular chaperones of the heat shock protein 70 (Hsp70) family play a crucial role in the presentation of exogenous antigenic peptides by antigen-presenting cells (APCs). In a combined biochemical and immuno-logical approach, we characterize the biochemical interaction of tumor-associated peptides with human Hsp70 and show that the strength of this interaction ...
01-Mar-2009
www.nature.com, online article
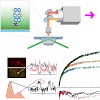
The molecular chaperone heat-shock protein 90 (Hsp90) is one of the most abundant proteins in unstressed eukaryotic cells. Its function is dependent on an exceptionally slow ATPase reaction that involves large conformational changes. To observe these conformational changes and to understand their interplay with the ATPase function, we developed a single-molecule ...
23-Feb-2009
ChemBioChem, online article
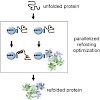
Matrix refolded: The formation of inclusion bodies, which are amorphous aggregates of misfolded insoluble protein, during recombinant protein expression, is one of the biggest bottlenecks in protein science. We report a stepwise, rational optimization procedure for refolding of insoluble proteins (see scheme). In comparison to refolding in-solution, this ...
22-Feb-2009
www.nature.com, online article
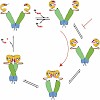
The molecular chaperone heat-shock protein 90 (Hsp90) couples ATP hydrolysis to conformational changes driving a reaction cycle that is required for substrate activation. Recent structural analysis provided snapshots of the open and closed states of Hsp90, which mark the starting and end points of these changes. Using fluorescence resonance energy transfer ...
19-Feb-2009

Many metabolic processes essential for plant viability take place in mitochondria. Therefore, mitochondrial function has to be carefully balanced in accordance with the developmental stage and metabolic requirements of the cell. One way to adapt organellar function is the alteration of protein composition. Since most mitochondrial proteins are nuclear encoded, ...
05-Feb-2009
Cellular and Molecular Life Sciences, online article

Chloroplast and mitochondria, the two organelles with an accepted endosymbiotic origin, have developed multiple translocation pathways to ensure the subcellular allocation of proteins synthesized by cytosolic ribosomes, and to guarantee their assembly into functional complexes in coordination also with organellar-encoded subunits. The evolution of different ...
31-Jan-2009
FEBS Journal, online article

It is widely accepted that chloroplasts derived from an endosymbiotic event in which an early eukaryotic cell engulfed an ancient cyanobacterial prokaryote. During subsequent evolution, this new organelle lost its autonomy by transferring most of its genetic information to the host cell nucleus and therefore became dependent on protein import from the cytoplasm. ...
23-Jan-2009

Recent advances have led to insights into the structure of the bacterial ribosome, but little is knownabout the 3D organization of ribosomes in the context of translating polysomes. We employed cryoelectron tomography and a template-matching approach to map 70S ribosomes in vitrified bacterial translation extracts and in lysates of active E. coli spheroplasts. In ...
14-Jan-2009
Molecular Biology of the Cell, online article

Transport of essentially all matrix and a number of inner membrane proteins is governed, entirely or in part, by N-terminal presequences and requires a coordinated action of the translocases of outer and inner mitochondrial membranes (TOM and TIM23 complexes). Here, we have analyzed Tim50, a subunit of the TIM23 complex that is implicated in transfer of ...
08-Jan-2009
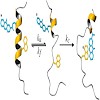
Coupling fast triplet–triplet energy transfer (TTET) between xanthone and naphthylalanine to the helix–coil equilibrium in alanine-based peptides allowed the observation of local equilibrium fluctuations in α-helices on the nanoseconds to microseconds time scale. The experiments revealed faster helix unfolding in the terminal regions compared with the central ...



 an Popov-
an Popov- eleketi
eleketi , Koyeli Mapa, Lada Gevorkyan-Airapetov, Keren Zohary, Kai Hell, Abdussalam Azem,
, Koyeli Mapa, Lada Gevorkyan-Airapetov, Keren Zohary, Kai Hell, Abdussalam Azem, 






